We have extensive experience taking research projects from early proof of concept and feasibility through scale-up and onto clinical manufacture for a range of dosage forms.
Formulation development expertise you can trust
With extensive experience in formulation development and dosage form optimisation, our experts can support you in accelerating First in Human (FIH) studies and towards clinical trials.
Through a science-led approach, we adapt and problem-solve to meet your timescales and patient targets.


Our approach
Working closely with the client at all stages, we start the development process by defining the project aims and understanding your target product profile.
Early on, we undertake pre-formulation activities, including characterising the active ingredient. Our experienced formulators then select excipients to develop the target dosage form. At all stages, we consider the suitability of the formulation, process for scale-up and ultimate commercialisation. Stability studies are a fundamental component of development work, with extensive analytical techniques available to characterise produced powders and dosage forms.
Once a suitable formulation has been developed, we will work to get the process to the target scale in our pilot plant which contains equipment that mirrors our GMP facility; providing confidence in the process before committing to clinical manufacture.
Dosage form development and manufacturing
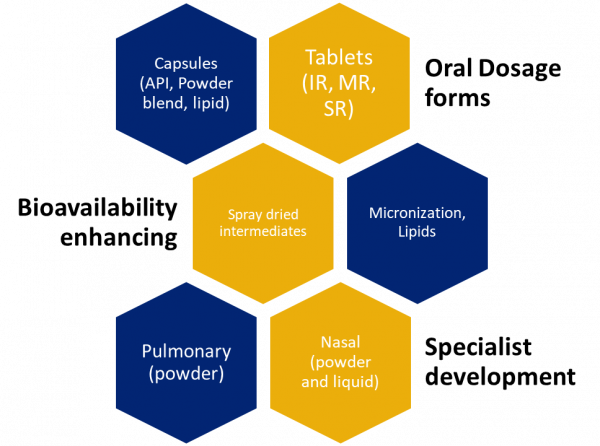
Experts in nasal, oral and pulmonary development. Our team harnesses advanced techniques and analytical skills to craft diverse dry dosage forms and innovative nasal and pulmonary deliveries. Our capabilities include:
Oral dosage forms
- Blending, dry granulation, milling, coating
- Tablets (IR, MR, Minitabs), capsule
- Small molecules, biologics, NCEs
- Grams to 250Kg batch sizes
Nasal and Pulmonary dosage forms
- Powder and liquid dosage forms
- Spray drying, micronization
- Capsule filling, device filling
- Blister packaging

Frequently Asked Questions
What is pharmaceutical spray drying, and how does it work?
Pharmaceutical spray drying is a process that converts a liquid solution or suspension into a dry powder form. It involves atomizing the liquid into fine droplets and then rapidly drying these droplets by exposing them to a stream of hot air or gas, causing the solvent to evaporate and leaving behind solid particles.
What are the advantages of using spray drying in pharmaceutical formulations?
Spray drying offers several advantages in pharmaceutical formulations, including precise control over particle size and distribution, improved solubility of poorly soluble drugs, enhanced bioavailability, ease of scale-up, and versatility in producing various dosage forms such as powders, granules, and microspheres.
What types of pharmaceutical products can be produced using spray drying?
Pharmaceutical spray drying can be used to produce a wide range of products, including inhalable powders for pulmonary and nasal delivery, solid dispersion systems (SDDs) to improve drug solubility, modified release formulations for controlled drug release, and stable formulations of biologics.
A CDMO like no other.
Why choose Upperton?
Expertise
Our Leadership team has extensive collective experience in steering products from pre-clinical to commercial manufacture.
Science-led
Our approach is scientific, collaborative and open, ensuring an open dialogue and transparency that fits your project.
Award-winning
We are a leading CDMO recognised for numerous awards including a King's Award For Enterprise.
Nimble
We adapt to your project needs, and pledge to never relegating it to the bottom of the list.

