September 15th 2022 : Newsletter
Successful nasal drug delivery of dry powder formulations requires a delivered dose that can penetrate beyond the nasal entrance region and deposit in the peripheral nasal cavity, whilst avoiding deeper penetration to the airways of the lungs. This delivery, or deposition pattern, is determined by the aerodynamic particle size of the powder exiting the device, which in turn is determined by the properties of the formulated product and the device used to deliver the dose. The geometric particle size of the formulated drug substance can be readily determined using a range of different particle sizing apparatus, for example laser-based particle sizers such as those produced by Sympatec or Malvern can quickly generate particle size distributions for powders as small as one micron, up to one millimetre, in a fast and reproducible manner. Whilst traditional particle sizing methods are important, such measurements do not necessarily provide evidence of how the particles behave aerodynamically. Nasal deposition will also be heavily affected by other powder properties such as density, rheology and cohesiveness which will all impact where in the nasal airways the powder will deposit and in what proportions. The importance of determining nasal delivery performance Generating data on the aerodynamic performance of powders delivered using nasal devices is important, just as in the delivery of dry powder formulations to the lung using traditional dry powder inhalers. Performance assessment should consider the following:
 The AINI accurately mimics deposition behaviour in each nasal region, allowing the collection of drug samples deposited in corresponding fractions of the nose for analysis. It is routinely fitted to the Copley NGI to capture any powder that passes through the AINI, and represents lung deposition.The AINI consists of a number of aluminium parts that can be assembled/dis-assembled with four specific deposition areas which correspond to the key areas for nasal deposition, namely:
The AINI accurately mimics deposition behaviour in each nasal region, allowing the collection of drug samples deposited in corresponding fractions of the nose for analysis. It is routinely fitted to the Copley NGI to capture any powder that passes through the AINI, and represents lung deposition.The AINI consists of a number of aluminium parts that can be assembled/dis-assembled with four specific deposition areas which correspond to the key areas for nasal deposition, namely: For more information on nasal device performance testing, or if you would like us to assess the performance of your dry powder formulation in a range of nasal devices, contact us for a discussion. Get in touch Upperton is a reactive and flexible CDMO that can, within days of initial contact, start feasibility studies with only a few milligrams of a client’s drug candidate. From the beginning, our clients will talk to an expert scientific team, who will manage projects with a science-led approach. Visit our website, https://upperton.com/ to find out more about Upperton. If you’re in need of a flexible CDMO, please contact us to find out how we can help you!
For more information on nasal device performance testing, or if you would like us to assess the performance of your dry powder formulation in a range of nasal devices, contact us for a discussion. Get in touch Upperton is a reactive and flexible CDMO that can, within days of initial contact, start feasibility studies with only a few milligrams of a client’s drug candidate. From the beginning, our clients will talk to an expert scientific team, who will manage projects with a science-led approach. Visit our website, https://upperton.com/ to find out more about Upperton. If you’re in need of a flexible CDMO, please contact us to find out how we can help you!

Aerodynamic testing of nasal powders; The Alberta Idealized Nasal Inlet (AINI)
To assist researchers and developers in testing nasal drug products, the Aerosol Research Laboratory of Alberta has recently introduced the Alberta Idealized Nasal Inlet (AINI). The design was based on rigorous numerical simulations and in vitro experiments, the results of which have shown that the AINI can accurately mimic regional deposition of nasal drug products that occurs in human nasal airway geometries. Whilst the AINI concept was originally conceived, designed and built at the University of Alberta, it is now being commercialised by Copley Scientific.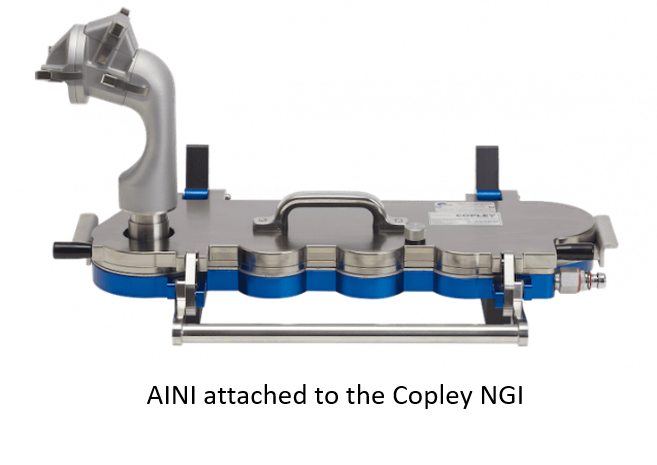 The AINI accurately mimics deposition behaviour in each nasal region, allowing the collection of drug samples deposited in corresponding fractions of the nose for analysis. It is routinely fitted to the Copley NGI to capture any powder that passes through the AINI, and represents lung deposition.The AINI consists of a number of aluminium parts that can be assembled/dis-assembled with four specific deposition areas which correspond to the key areas for nasal deposition, namely:
The AINI accurately mimics deposition behaviour in each nasal region, allowing the collection of drug samples deposited in corresponding fractions of the nose for analysis. It is routinely fitted to the Copley NGI to capture any powder that passes through the AINI, and represents lung deposition.The AINI consists of a number of aluminium parts that can be assembled/dis-assembled with four specific deposition areas which correspond to the key areas for nasal deposition, namely:- The vestibule (nostril); where blood flow is lower and the potential for systemic delivery is low
- The turbinates; highly vascularised membranes, priority target for systemic delivery and for localised immunisation
- The olfactory region; rich in blood vessels and site for the optic nerves to reach the brain. Believed to be the primary target for nose-to-brain delivery
- The nasopharynx; the area at the top of the throat prior to the windpipe.
 For more information on nasal device performance testing, or if you would like us to assess the performance of your dry powder formulation in a range of nasal devices, contact us for a discussion. Get in touch Upperton is a reactive and flexible CDMO that can, within days of initial contact, start feasibility studies with only a few milligrams of a client’s drug candidate. From the beginning, our clients will talk to an expert scientific team, who will manage projects with a science-led approach. Visit our website, https://upperton.com/ to find out more about Upperton. If you’re in need of a flexible CDMO, please contact us to find out how we can help you!
For more information on nasal device performance testing, or if you would like us to assess the performance of your dry powder formulation in a range of nasal devices, contact us for a discussion. Get in touch Upperton is a reactive and flexible CDMO that can, within days of initial contact, start feasibility studies with only a few milligrams of a client’s drug candidate. From the beginning, our clients will talk to an expert scientific team, who will manage projects with a science-led approach. Visit our website, https://upperton.com/ to find out more about Upperton. If you’re in need of a flexible CDMO, please contact us to find out how we can help you!
