PulmoCraft™
PulmoCraft™ a Manufacturing Solution for DPI Dosage Forms.
Drug delivery into the lung is one of the fastest growing, yet technically challenging areas of pharmaceutical development. Producing a successful pulmonary drug product requires expertise in formulation development, particle engineering and device selection. Inhalation product testing capabilities are also vital, to ensure that the developed product can effectively deliver the target dose into the correct region of the lung.
Development of Pulmonary Products
When developing a pulmonary drug product, the first key decision will be whether the product should be a Liquid or Dry Powder formulation. This choice will be dependent on several factors including speed to clinic, solubility and stability profile of the API, and the target dose to be delivered.
Drug product development includes two equally important aspects:
Formulation Development: Producing a stable dry powder or liquid formulation, with the correct physical properties for successful delivery, such as viscosity, for liquid formulations, or particle size for dry powder formulations.
Delivery Performance: Ensuring that the target delivered dose is achieved from the selected device and that the aerodynamic performance will result in the deposition in the target region of the lung.
Delivery to the Lung: Dry Powder Inhalers (DPI)
Successful development of a DPI dosage form requires a good understanding of the physiochemical properties of the API, extensive testing of the dry powder formulation, and its’ performance when delivered from the selected device.
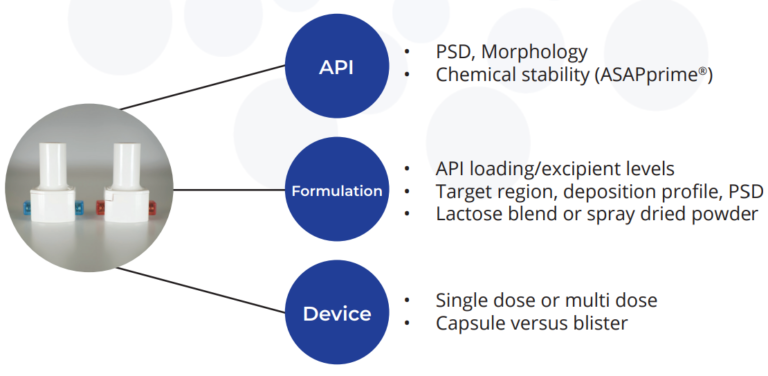
The key to the successful development of a DPI formulation is the development of formulated drug substance particles with the correct aerodynamic size for pulmonary delivery. At Upperton this is achieved through two basic approaches: Pharmaceutical Spray Drying and Jet Milling.
Pharmaceutical Spray Drying is one of the most flexible and versatile particle engineering techniques and Upperton has over twenty years of experience in developing formulations and spray drying processes to create microparticles with the correct aerodynamic properties for deep lung delivery.
Once optimised spray drying processing parameters have been defined, the Upperton spray dryers can produce kilo quantities of engineered microparticles that can be precision filled into capsules or directly into a DPI.
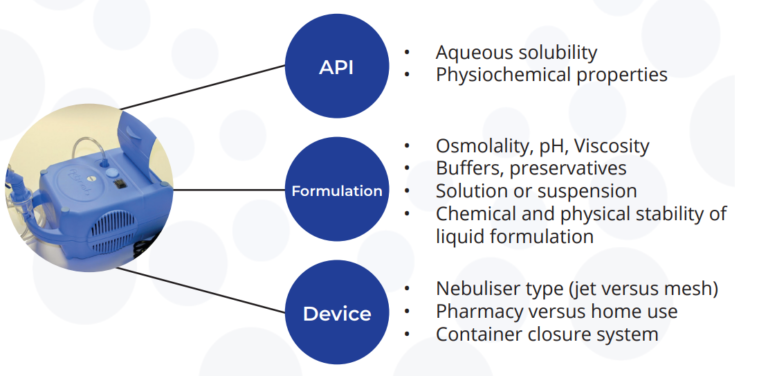
Delivery to the Lung: Liquid Formulations
Many customers initially enter the clinic with a liquid formulation for delivery via a nebuliser. The use of a liquid formulation is often seen as a ‘quick’ route into clinic as it avoids the need for more extensive dry powder formulation development timelines.
Successful development of liquid (nebulised) formulations requires a thorough investigation into the properties of the API, the formulated liquid product and the performance of the formulation in the device.
Analytical Testing
Generating stability and performance data on both liquid and dry powder pulmonary dosage forms require significant know-how and technical capability. Meaningful and reproducible performance data is critical to successful product development.
Upperton has a range of analytical capabilities to support development activities, including particle size by laser diffraction, spray pattern and plume geometry, microscopy including scanning electron microscopy, aerodynamic particle size distribution by cascade impactor (NGI), emitted dose and content uniformity.

