Pharmaceutical Services
Upperton provides you with a comprehensive CDMO services package, enabling your molecule to progress from early feasibility and dosage form selection, to formulation development and ultimately manufacture of the final dosage form to support clinical testing in humans.
To achieve our goals, we offer our clients a formulation development that is led by science and backed up by the ‘know-how’ gained from over twenty years of CDMO services in the industry. Our knowledge spans basic formulation development through process scale-up and the eventual transfer into GMP manufacture.
Our development activities are supported by comprehensive analytical capabilities to enable us to support the seamless development of our client’s product towards the clinic.
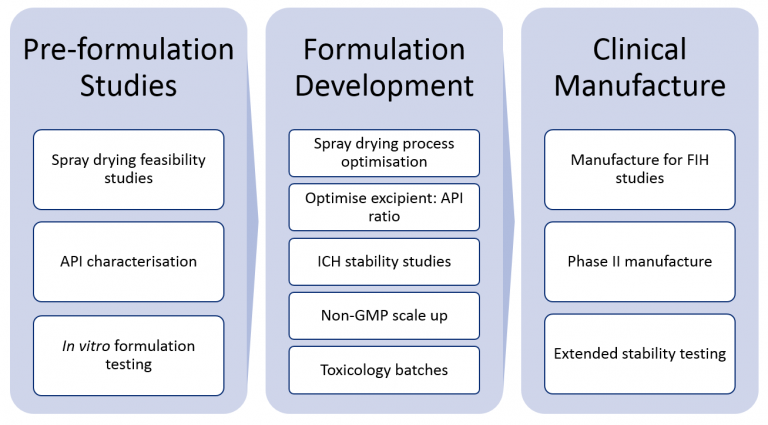
Our clients join us at varying stages of the development pathway. No client is typical and all have their unique challenges. Whatever the challenge, we offer our clients a coherent transition into the clinic.
We Can Help You Through This Whole Path or Any Individual Stages
A client’s journey with Upperton might include:
- Feasibility study; ensuring your API can be spray dried and optimise the spray drying parameters.
- Formulation development; choosing the dosage form to allow efficient delivery of API into the patient.
- An ICH stability study; ensures formulation is suitable for storage.
- Scale-up studies; using proven models, define increased batch size methods to support development studies and for transfer into GMP.
- GMP clinical manufacture of formulation up to clinical phase III.
Our scientists recognise that successful development programmes begin with a thorough understanding of the physico-chemical characteristics of the API, and any potential stability or excipient incompatibility challenges.
Formulation Development
Using a range of techniques including, DSC / TGA, XRPD, Microscopy, FTIR, Particle sizing. etc. we gain fundamental understanding and insight into the behaviours and of your API and support the development activities required to support First in Human studies and product development.
- Material Identification
- Solubility Screening
- Excipient Compatibility
- Physio Chemical characterisation
- Support or enabling protocols UpperSolv™, PulmoCraft™ and UpperNose™


Phase 1 to Phase 3 Clinical Supply
Once your product enters clinical development our dedicated team of analytical experts work with you and our cross functional teams to transfer, development, and phase appropriate validation of your analytical methods and performance of QC and release testing including:
- Assay, Content uniformity and related substances by HPLC
- Performance testing including Dissolution and Disintegration
- Moisture and residual solvents
- IC Stability testing
- Cleaning verification
- DoE studies to investigating critical process parameters
Process Scale-Up and Optimisation
Using a range of techniques including, DSC / TGA, XRPD, Microscopy, FTIR, Particle sizing. etc. we gain fundamental understanding and insight into the behaviours and of your API and support the development activities required to support First in Human studies and product development.
- Material Identification
- Solubility Screening
- Excipient Compatibility
- Physio Chemical characterisation
- Support or enabling protocols UpperSolv™, PulmoCraft™ and UpperNose™


Registration and Regulatory Affairs Activities
Once your product enters clinical development our dedicated team of analytical experts work with you and our cross functional teams to transfer, development, and phase appropriate validation of your analytical methods and performance of QC and release testing including:
- Assay, Content uniformity and related substances by HPLC
- Performance testing including Dissolution and Disintegration
- Moisture and residual solvents
- IC Stability testing
- Cleaning verification
- DoE studies to investigating critical process parameters
Analytical Method Development, Validation and QC Testing
Using a range of techniques including, DSC / TGA, XRPD, Microscopy, FTIR, Particle sizing. etc. we gain fundamental understanding and insight into the behaviours and of your API and support the development activities required to support First in Human studies and product development.
- Material Identification
- Solubility Screening
- Excipient Compatibility
- Physio Chemical characterisation
- Support or enabling protocols UpperSolv™, PulmoCraft™ and UpperNose™


